The Oncology Center of Excellence (OCE) Real-time Oncology review (RTOR) Pilot program was initiated in February 2018, specifically for supplemental oncology new drug applications (NDA)s and biologic license applications (BLAs) of previously approved drug products. Beginning in December 2018, the RTOR program was extended to select original NDAs for new molecular entities (NMEs) submitted under section 505(b) of the Federal Food, Drug, and Cosmetic Act (FD&C Act), and original BLAs submitted under section 351(a) of the Public Health Service Act.
The RTOR program was designed to streamline the review of promising new oncology treatments, while allowing Sponsors an earlier application review start. This provides an opportunity for early Agency engagement to proactively identify potential deficiencies and review top line clinical data upon availability as opposed to following the completion of a final clinical study report.
While this approach results in shorter development time overall, it can result in a more complex race for sponsors, as the distance from the “top line” starting line to the finish is considerable and includes some unique hurdles.
Eligibility
According to the FDA’s final Guidance issued in November 2023, a submission must demonstrate the following to be eligible for the RTOR program:
- clinical evidence from well-controlled investigation(s) that indicates substantial improvement on a clinically relevant endpoint (s) over available therapies,
- easily interpretable clinical trial endpoints, such as progression free survival (PFS) and overall survival (OS), and
- no aspect of the submission is likely to require a longer review time, such as a new Risk Evaluation and Mitigation Strategy (REMS) or an advisory committee review.
RTOR Submission Process
The Sponsor may apply for the RTOR program as early as 2-4 weeks after the patient database lock of top line results from their pivotal trial and receive a decision within 20 business days. Upon acceptance into RTOR, the applicant may meet with the OCE and the appropriate review division via a teleconference to align on a RTOR plan that includes the proposed timeline and components of the application.
The FDA has provided a typical content plan and timeline for the RTOR pre-submission package as shown below:
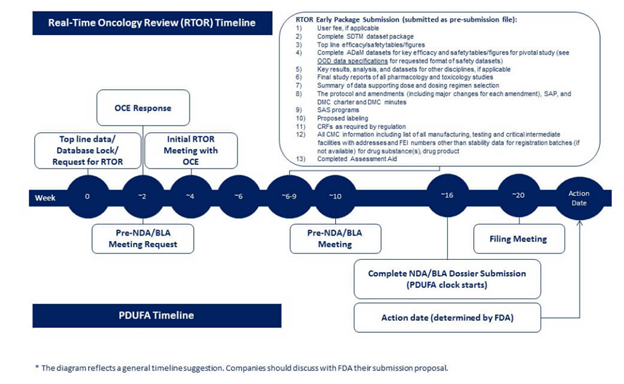
Figure 1. General RTOR Timeline
Image source: FDA website (Real-Time Oncology Review | FDA)
RTOR Review Process
As the RTOR program does not require complete modules to be submitted prior to the full application, this offers an advantage over existing mechanisms for rolling review. The Sponsor submits components as they become available, with three pre-submissions and a final submission.
Pre-submissions may include CMC information, statistical programs, proposed labeling and key results for other disciplines, prior to the final submission including the final clinical study reports. Throughout this process, the agency may communicate preliminary review issues or questions that will need to be addressed prior to the complete marketing application, which includes any remaining components that were not previously submitted.
Challenges of a Rolling Submission
One of the main challenges of the RTOR process is the management of the Information Requests (IRs) from the FDA in parallel with ongoing submission planning. The frequent flow of IRs also involves tight turnaround times, typically within 24 to 48 hours.
These IRs may also impact future components of the rolling submission, which will need to incorporate the FDA’s feedback. To stay on top of these aggressive timelines and rapid review cycles, partner with an experienced team who can coordinate cross-functional communication, drive project deliverables around the clock, and manage submission risks.
How MMS can help you in your RTOR submission planning and responding to IRs
The FDA evaluated the approval time for oncology applications and found that those using the RTOR process had a shorter median submission review to approval time of 3.3 months. Our team of industry experts has experience with expedited application submission and review approaches and can guide you from top line to finish by:
- Seeking alignment with the FDA on a feasible RTOR plan accounting for aggressive timelines
- Optimizing cross-functional planning for rapid, compliant and complete submissions
- Executing your program-specific data management plan
- Preparing your team for IR responses in a timely fashion
Operational Considerations to Ensure Success
Here are a few key regulatory operations recommendations for working with large oncology study programs submitted under RTOR:
- Finalize nonclinical study module data early to include it in the first pre-submission.
- Have your programming team review the acceptable dataset formats to ensure they stay within specified file size limits for large datasets. For non-standard datasets, providing a define.pdf and a reviewer’s guide is suggested for better traceability.
- Author and reference consistently to avoid resubmission of data.
- Establish a consistent and standard presentation of case report forms across the clinical study reports
- Develop an Assessment Aid document to summarize the critical information in the application to optimize the agency review.
- Adopt rolling publishing and review of submissions to ensure the critical timelines are met.
For questions regarding rapid access to cancer treatments under the RTOR process, please click here and we will connect you with the appropriate expert.
References
- Real-Time Oncology Review | FDA
- Guidance for Industry (fda.gov)
- Priority Review | FDA
- de Claro RA, Gao JJ, Kim T, Kluetz PG, Theoret MR, Beaver JA, Pazdur R. U.S. Food and Drug Administration: Initial Experience with the Real-Time Oncology Review Program. Clin Cancer Res. 2021 Jan 1;27(1):11-14.
Authored by:
- April Nguyen, Senior Global Regulatory Affairs Manager at MMS Holdings
- Ben Kaspar, Regulatory Affairs and Strategic Consulting Senior Director at MMS Holdings
- Swathi Pandhiti, Regulatory Operations Associate Director at MMS Holdings
- Raksha Srinivas, Regulatory Operations Team Lead at MMS Holdings





