Data Science
Experts in data science achieve new levels of insight through real world data (RWD), advanced analytics, and interactive visualizations.
Sponsors can benefit from greater efficiency during clinical development, analysis of data for marketed products, and reduction in duration and number of clinical trials.
The Data Science experts at MMS open new possibilities for sponsors searching for data-driven insights for pharmaceutical, biotech, and healthcare organizations. The team employs a wealth of regulatory expertise, strong processes, and purpose-built, flexible technology to drive efficiency using real world data (RWD). This leads to uncovering new insights on disease, compound, patient, and possible operational improvements for those involved in life-sciences and research.
When engaging MMS, Sponsors have gained immense value, including but not limited to:
- Efficiency in clinical development, using near real-time, interactive data visualization to drive program-critical decisions
- Reimagined solutions for complex regulatory questions, through the combined use of industry expertise, strong processes, and integrated technology (read the case study)
- Curating non-traditional data sources and building a framework for exploration, leading to new treatments and label expansion
- Leveraging the expertise of MMS data scientists to support data-driven objectives through project based or FSP models
The sections below detail comprehensive Data Science solutions, including consulting, technology, and functional solutions staffing. Request a meeting to connect with a Data Science expert and learn why MMS is the Data Science partner of choice for innovative life science teams and organizations.
Planning & Consulting
Data Science discovery is foundational for planning the use of real world data to answer organizational objectives and improve business decisions. Through a purposeful approach, our experts will work to define the strategic goals for a data science engagement, including identification of appropriate data to pair with data available from clinical trials.
In the tree diagram below, Sponsors can view how RWD flows from the roots into useable insights and real world evidence (RWE) at the leaves.
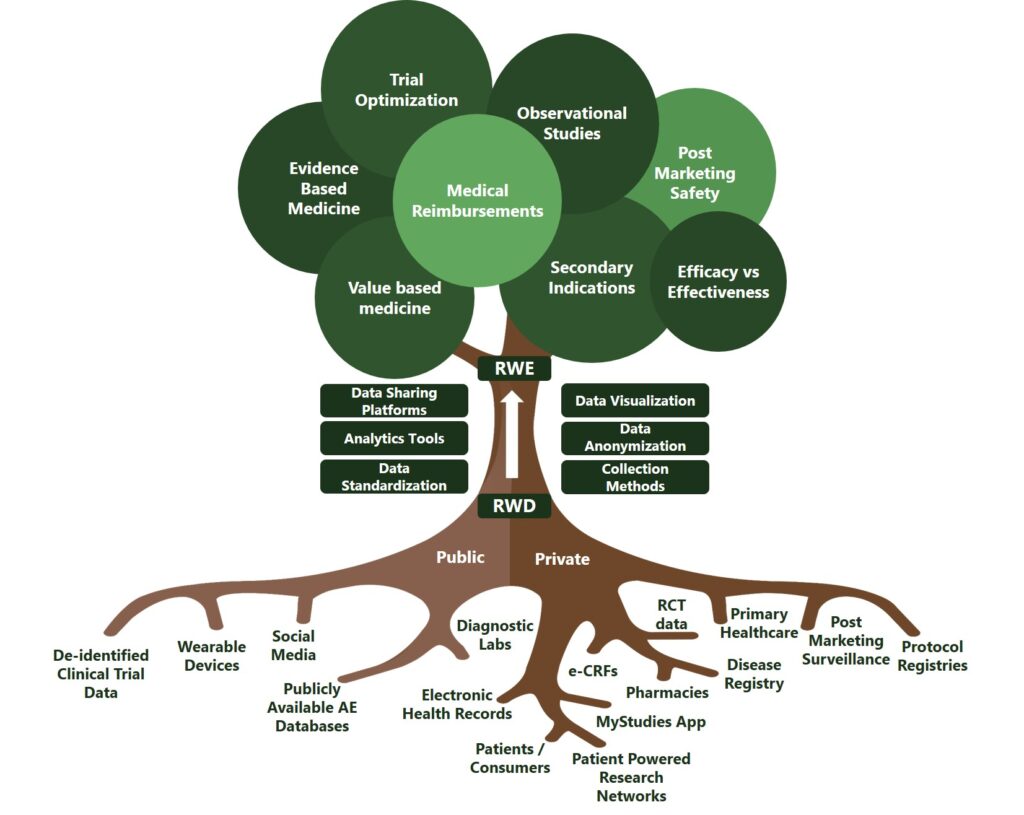
Image not for reuse without permission of MMS.
Data Curation
Data Scientists at MMS are proficient in the abstraction and preparation of data from any source for analysis and/or visualization. At times, this may require creation of a database or data lake to store data, with sources that may include Electronic Health Records (EHR), wearables or other health devices, social media, medical claims, and others.
Here are a few ways that Sponsors can engage MMS:
- Data Curation, including relationships with data brokers/source(s) to collect and prepare for analysis
- Intake and standardization of streaming data (i.e. wearables)
- Curation of EHR data for retrospective analysis to support label expansion
To aid in data curation, MMS utilizes Datacise CURATE, a flexible cloud-based application for the collection and aggregation of clinical data through a variety of data mapping or abstraction methods.
Advanced Analytics
Data Scientists at MMS can provide Sponsors with a deeper understanding of data through custom algorithms and advanced analytical models. This support may include machine learning (ML), natural language processing (NLP), and data mining or web scraping. Our approach allows for complex statistical methodology and novel programming languages, including Julia, Python, R, and others.
Here are a few ways that Sponsors can engage MMS:
- Natural language processing of Twitter and Reddit posts to extract drug product related content for safety surveillance
- ML-based detection of clinically important subgroups (i.e. data mining)
- Location-based analyses (i.e. GPS and wearables) and compliance reporting
- Image recognition and analysis
- Complex exploratory models
To aid in advanced analytics, MMS utilizes Datacise ANALYZE, a fully-integrated cloud-based technology platform that supports advanced analytical needs for RWD and other data.
Data Visualization
Data Scientists at MMS provide Sponsors with rapid data insights through browser-based interactive dashboards. With the potential for API data extraction into dashboards, Sponsors can take advantage of automated data refreshes, reports, and alerts to ensure that data is always up-to-date.
Here are a few ways that Sponsors can engage MMS for visualization support:
- DSMB and Adjudication meetings including dashboards to augment static tables and listings
- Patient profiles to aid in ongoing safety reviews and assessments
- Dashboards for medical device and wearables data
- Site performance dashboards that help clinical teams and data managers monitor study progress
- Safety surveillance signal detection, using social media and regulatory data sources (i.e. FAERS)
To aid in data visualization, MMS utilizes Datacise EXPLORE, a fully-integrated cloud-based technology platform that provides integrated data visualizations and dashboards to support analysis, review, and ultimately speed decision-making.
Functional Service Provision (FSP) Support Models
Sponsors around the globe engage with MMS for FSP partnerships in the area of Data Science. Our FSP models are designed to be flexible and scalable, allowing our team and level of engagement to smoothly shift with your changing needs. Key benefits to engaging in an FSP model with our data scientists include:
- Experience and consistency of an FSP relationship allows MMS to bring the greatest value, rather than other transactional models
- Effective and efficient communication plans by defining roles and responsibilities, with a clear escalation process
- Gain efficiencies through interdependent cross-functional services
- Through value-added FSP partnerships, MMS strives for seamless collaborations that build trust
Data Science Partnerships
 Along with MIT, the Center for Translational Medicine at the University of Maryland, and Julia Computing, MMS is a co-founding member of the Health Analytics Collective – a pioneering group that strategically uses real world evidence in drug development. The collective brings proven regulatory expertise, strong processes, and the latest technology to provide data-driven insights, with a goal to shorten drug development timelines of new treatments.
Along with MIT, the Center for Translational Medicine at the University of Maryland, and Julia Computing, MMS is a co-founding member of the Health Analytics Collective – a pioneering group that strategically uses real world evidence in drug development. The collective brings proven regulatory expertise, strong processes, and the latest technology to provide data-driven insights, with a goal to shorten drug development timelines of new treatments.
Learn more about the collective
MMS is currently open to speaking with potential partners to acquire RWD sources to further expand our technology-enabled Data Science solutions. If you would like to discuss further, fill out the partnership form on this page.
Innovation in Data Science
Data has never been easier to visualize
Datacise®  is an innovative, cloud-based technology platform, with the ability to aggregate and mine data to provide real-time insights for our Sponsors. With this secure, scalable data science platform, Datacise® allows Sponsors to immediately analyze and visualize clinical data, Real-World Data (RWD) and data from other data sources.
is an innovative, cloud-based technology platform, with the ability to aggregate and mine data to provide real-time insights for our Sponsors. With this secure, scalable data science platform, Datacise® allows Sponsors to immediately analyze and visualize clinical data, Real-World Data (RWD) and data from other data sources.
client quote
“The Dashboards are very nice. They are a major step forward in looking at safety data and exploring it.”
-Head of Clinical Development, Top 10 Pharma
Related Client Case Studies
RWE to Support an Orphan Drug Application
 Learn the story of a sponsor who engaged MMS to identify prevalence for a subset of a disease population and how MMS exceeded expectations, using non-traditional data and real world evidence to complete an accurate health authority response in time.
Learn the story of a sponsor who engaged MMS to identify prevalence for a subset of a disease population and how MMS exceeded expectations, using non-traditional data and real world evidence to complete an accurate health authority response in time.
Speak with a Data Science Expert

