Good Laboratory Practice (GLP) is a quality control system for clinical research laboratories and organizations to ensure the integrity, consistency, and reproducibility of the data within non-clinical and clinical studies of products in development for human or animal health.
It is important for a clinical trial sponsor to be aware of which stages in the drug development process need to be Good Laboratory Practice (GLP) complaint in order to maintain a smooth process. Additionally, less challenges will be encountered when deciding to switch from a non-GLP to GLP study.
What is the difference between GLP and non-GLP studies?
There is a clear distinction between Good Laboratory Practice (GLP) and Non-GLP studies and it is based on the level of compliance with applicable regulations. Good Laboratory Practice (GLP) studies are inspected by the FDA, whereas non-GLP studies are not.
Good Laboratory Practice (GLP) studies may increase the cost of a study, particularly when considering and/or conducting validation processes involving extensive operational efforts to define the use of appropriate operating procedures, methods, and equipment that will eventually become GLP relevant.
Considerations to the need and extent of validation during the earlier stages of drug development will have high impact during the later stages of the drug development process.
Do this to help ensure Good Laboratory Practice (GLP) compliance
To ensure Good Laboratory Practice (GLP) compliance, all work within laboratories should be conducted in accordance within the principles of GLP and performed by adequately-trained scientists.
Good Laboratory Practice (GLP) regulations are rooted in quality assurance and clinical study sponsors must show evidence of the safety in any clinical or exploratory studies. In accordance with the guidelines for Good Laboratory Practice (GLP) regulations, all equipment used to generate, measure, or assess data should undergo validation to ensure that particular equipment is of the appropriate design and capacity and that it will consistently function as intended.
Therefore, validation is considered to be an essential step when deciding to undergo Good Laboratory Practice (GLP) studies.
Validation of Good Laboratory Practice (GLP) studies
Validation encompasses activities at different stages in the drug development lifecycle and protocols/qualifications executed as part of the validation effort must be pre-approved prior to execution and post-execution by a Validation designee, Subject Matter Expert (SME) or System Owner, and Quality Assurance.
Validation activities may include the following:
- Facilities, equipment, and analytical methods known as qualification, which is carried out by conducting Design Qualification (DQ), Installation Qualification (IQ), Operational Qualification (OQ) and Performance Qualification (PQ)
- Computerized Systems Validation
- Processes Validation
- Cleaning Validation
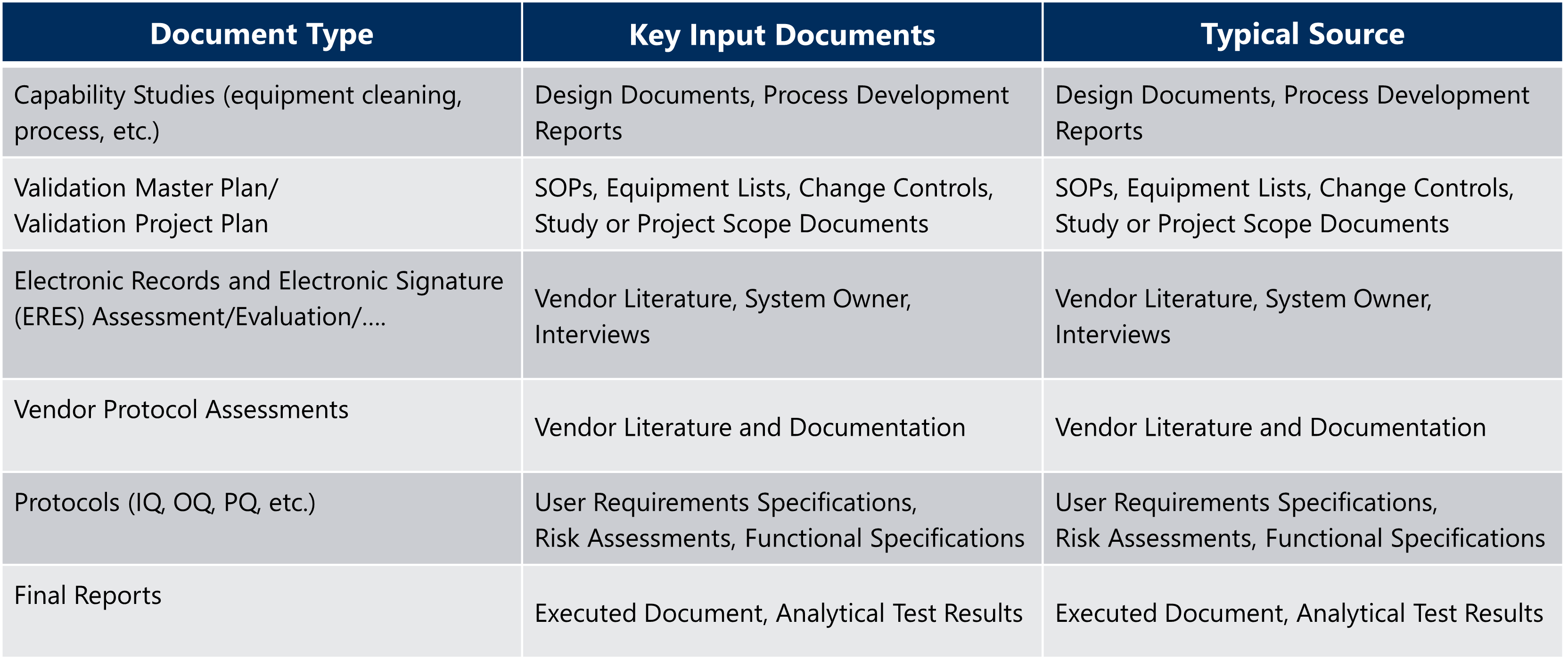
Table 1: Suggested Validation Documentation Guide
Performing retrospective validation
Particularly, for existing equipment or systems used during the earlier stages of drug development that are now part of a Good Laboratory Practice (GLP) study, a retrospective validation should be carried out. This should include testing and change control within the production environment.
Retrospective validation allows the company to identify any gaps within the validation deliverables and work a plan to complete and meet the level of compliance for a successful global health authority or regulatory inspection outcome. Validation specifies and coordinates activities to ensure compliance with regulatory requirements and the integrity of generated data.
Regardless of role, defining the objectives of the study to be conducted is key to identify the level of compliance. There are certain components of the clinical study that differ in deliverables and the need to evaluate based on the type of study being conducted. Understanding the details of the study and deliverables will simplify the Good Laboratory Practice (GLP) validation process and determine what efforts are needed to define the level of compliance for the study.
It’s important to choose an experienced and knowledgeable vendor partner that can help assess the quality management system of an organization for validation to help achieve the level of compliance for a successful FDA inspection outcome.
For further discussion, Please click here to be connected to a Quality and Compliance expert.
Authored by: Ileana Rivera; Senior Quality and Compliance Specialist, Quality and Compliance.





