Clinical trial deferrals are a growing topic among those in the transparency niche of the pharmaceutical industry, especially as all new and ongoing clinical trials within the European Union (EU) must now be uploaded to the Clinical Trial Information System (CTIS) starting 31 January 2023 through 30 January 2025 under the EU Clinical Trials Regulation (EU CTR). This requirement poses a significant challenge for many sponsors, as they must interpret the regulations accurately and act swiftly to meet regulatory timelines.
A question that many pharmaceutical sponsors are currently pondering is how to strategize regarding the clinical trial deferrals option being removed for in scope publication documents falling under the EU CTR.
In an update of revised transparency rules published by the European Medicines Agency in October 2023, sponsors were advised that documents that were once able to be deferred for publication for new clinical trial applications (CTAs) would not have the deferral option after the implementation of the revised transparency rules. Before the proposed revision of the transparency rules, an application for deferral of certain submission documents would allow the significant delay of their publication, dependent on the category and phase of the clinical trial, for years following the end of the trial in the EU.
The revised transparency rules are slated to be fully implemented 18 June 2024 (1), propelling the pharmaceutical industry to decide whether they should try to apply for a deferral or provide more heavily redacted documents and forego the application.
Sponsors must wrestle with the delicate balance of disclosing information in their submission documents that will not provide an edge to competitors and/or undermine their economic interests as a pharmaceutical company with the increased requirements of clinical trial transparency for the disclosure of submissions requested by global health authorities on their respective public websites.
Sponsors have been given the weighty task of trying to decide what company information within the submission documents may be publicly disclosed and what must remain confidential. The burden for new sponsor study teams with little or no experience in the identification and justification of company confidential information (known as CCI) may seem overwhelming.
What Is Known?
EU CTR requires that certain Part I and Part II documents be published on the public portal (2) necessitating redacted documents to be provided for decision for authorization of the trial in CTIS.
Pre-implementation
- Sponsors may continue to apply for deferral until the revised rules are implemented. EMA guidance has clearly stated that little to no CCI is to be redacted in the applicable documents when a deferral is requested in the initial CTA.
- All documents in the CTA submitted to the CTIS before the revised clinical trial transparency rules will not be disclosed to the public, regardless of the previous application for a ‘deferral’ or ‘for publication’ status.
Pre-implementation
- Before the revised transparency rules are fully implemented, sponsors may provide a version “for publication” and “not for publication” only for the documents in scope of the revised clinical trial transparency rules.
- Documents not included in the lists below for Categories 1-3, such as the investigator’s brochure, scientific advice letters, assessment reports, corrective measure documents, and interim summaries of results are out of scope for publication (3). The list of scope documents includes the following documents to be published for the CTA (excluding the Category 1 adult trials noted below):
-
- Category 1 trials[1] (3)
-
- Protocol, synopsis and patient facing documents upon results submission for pediatric trials if available
- Final summary of results, with a layperson summary as soon as submitted
- Clinical study report 30 days after marketing decision
Note:
No deferral is available for Category 1 pediatric trials;
Deferral for publication may be applied for up to 30 months after the end of the trial in the EU/EEA for Category 1 adult population trials for the documents above.
-
- Category 1 trials[1] (3)
-
- Category 2 and 3 trials[2],[3] (3)
-
- Protocol, synopsis and patient facing documents, first Member States Concerned (MSC) decision
- Summary of medicinal product characteristics (SMPC), at first MSC decision
- Informed consent form and patient sheet at respective MSC decision
- Recruitment arrangements, including procedures for inclusion and copy of advertising material at respective MSC decision
-
- Category 2 and 3 trials[2],[3] (3)
1Category 1 trials include Phase 0, 1, Pharmacokinetics/Pharmacodynamics, Bioavailability, Bioequivalence, Biosimilarity trials, and Similarity trials for biosimilar product.
2Category 2 trials include Phase 1 and Phase 2 integrated clinical trial Phase 2 and 3 trials.
3 Category 3 trials include Phase 3 and Phase 4 integrated clinical trials, Phase 4, and low interventional clinical trials.
-
-
- Final summary of results, with a layperson summary as soon as submitted
- Clinical Study Report (CSR) 30 days after marketing decision
-
- For the documents out of scope of publication under the revised transparency rules, a cover page waiving off the publication should be uploaded in the slot “for publication” for those documents. (4)
Post-Implementation
- The European Medicines Agency (EMA) has projected the timeframe for the full implementation of the revised transparency rules as 18 June 2024 (1). After the implementation of the revised transparency rules, following changes are likely to take place:
-
- Documents in scope for publication submitted in CTIS will be published at decision.
- No deferral option (except for Category 1 adult population trials) will be available to the sponsor, so study teams will have to consult on more heavily redacted documents for submission.
- Documents submitted for substantial modifications will be made public immediately, even if the historical trial had a deferral in place (e.g., the protocol amendment submitted for substantial modification will now be made public immediately under the new rules even though the protocol submitted under the deferral option was not subject to publication).
- Structured data fields for responses for information (RFI), and response documents to RFI will remain unpublished (4).
-
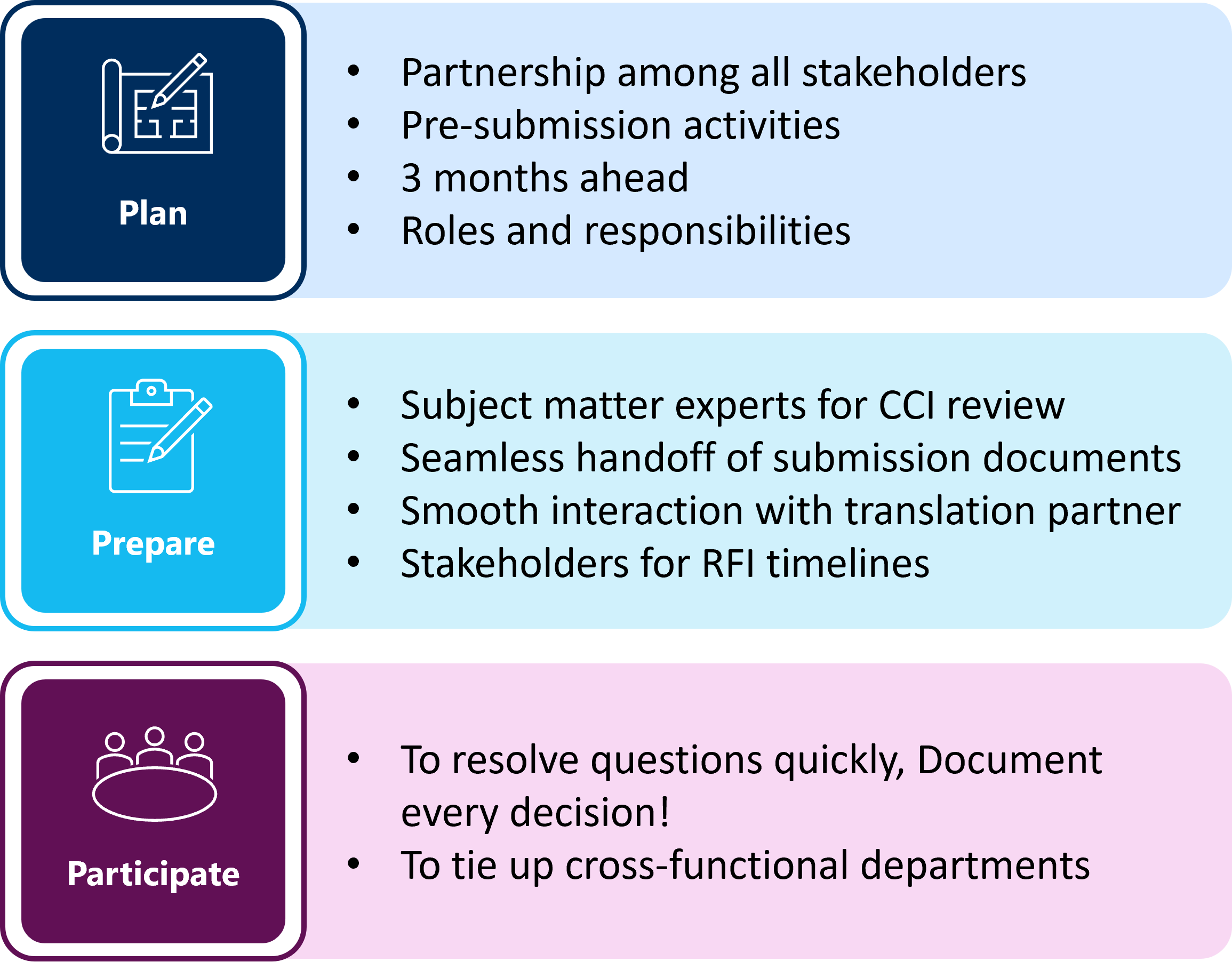
Plan, Prepare and Participate
The implementation of the revised clinical trial transparency rules on 18 June 2024 (1) will affect pharmaceutical sponsors in their decision-making during pre-submission activities. During this interim period prior to the full implementation of the revised clinical trial transparency rules, sponsors need to have a mindset of planning and preparing their teams and various departments for the changes at hand.
Sponsors are encouraged to make expedient decisions on whether to apply for the deferral of certain CTA documents while this approach remains an option. If submission timelines prohibit applying for a clinical trial deferral, the responsibility for the consistent redaction of the submission documents for both protected personal data (PPD) and CCI remains with the sponsor.
MMS offers bundled services, trainings to sponsor teams on the identification of CCI, consultation to ensure compliance, proactive planning, dedicated project teams to meet stringent EU-CTIS timelines and communicating effectively across departments to lead stakeholders. Planning, preparing, and participating in the process with experts provides support and ensures a pathway to a successful submission in CTIS. Find details on support and request a call here.
References
- Launch of revised CTIS transparency rules – European Union (europa.eu) Accessed 24 April 2024
- Search for clinical trials – EMA (euclinicaltrials.eu)
- https://phuse.s3.eu-central-1.amazonaws.com/Deliverables/Data+Transparency/EU+CTR+Update+–+Year+2.pdf Accessed 22 April 2024
- ACT EU_Q&A on protection of Commercially Confidential Information and Personal Data while using CTIS (europa.eu) Accessed 22 April 2024
Authored by:
Raina Agarwal, Associate Director, Clinical Trial Disclosure and Transparency, Regulatory and Medical Writing, and
Shawn Lantz, Senior Transparency Specialist, Transparency, Regulatory and Medical Writing





