These three words may be the magic formula that Sponsors will have to adopt when the European Clinical Trials Regulation comes into force.
Sponsors will have to prepare their timelines well to prevent enduring disappointments or ultimately delaying the start of a clinical trial in a European country or the application of a substantial modification.
Potential Scenario: One Year In
There is nothing better than a case study to illustrate the need for coordination. Let’s imagine it’s February 2023, for instance. The one-year transitional period is over, meaning the new EU Clinical Trials Regulation now applies to all new studies with no possibility to be ruled by the old EU Clinical Trial Directive anymore.
ABC Pharma (name created for the purpose of this scenario) has begun its clinical trial in the United States and wishes to open several centers in five European countries: France, Germany, Poland, Portugal, and Belgium.
Under the rules of the old EU Clinical Trials Directive, the Sponsor would have to go to each country to submit an administrative dossier to the National Competent Authority (NCA) and an ethics dossier to one or more ethics committees depending on the local requirements.
In this case, five administrative dossiers (1 per NCA) and at least five ethics dossiers are needed. More dossiers mean more possibilities to receive feedback and decisions from the NCAs and ethics committees.
The EU Clinical Trials Regulation will simplify this.
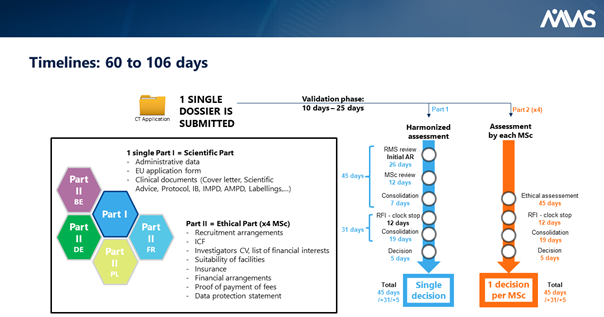
Under the rule of the new EU Clinical Trials Regulation, ABC Pharma will have to submit only one administrative dossier (called Part 1) and one ethics dossier per country (called Part 2). So, in our case study, one administrative dossier (Part 1) and four ethics dossier (Part 2) are required.
Part 1 and t 2 will be completed in the new EU Clinical Trial Portal. Part 1 will be assessed by the NCAs together.
As for Part 2, this is now the responsibility of each member state to organize its ethics committee in order to deliver only one ethical decision per country. In summary, when ABC Pharma clicks on the “submit” button, this action will trigger the assessment of the entire dossier (Part 1 and 2) by the five NCAs and the five ethics committees simultaneously. Within 60 to 106 days, ABC Pharma will receive clinical trial authorizations in the European countries with this unique procedure.
Potential problems in this scenario
This example is undoubtfully a simplification and a benefit for ABC Pharma, but they could also face some inconveniences.
Before clicking on the “submit” button, ABC Pharma should make sure that they are prepared to deal with the consequence of this simple click.
Once the dossier (Part 1 and 2) has been submitted, ABC Pharma will no longer be able to add a new EU country. The whole process will be frozen during the review period of Part 1. If the Sponsor wanted to add a new country, let’s say Denmark, for instance. The best-case scenario is a 60–day waiting period, and the worst-case scenario is a 106–day waiting period.
Only when ABC Pharma receives a favorable opinion on Part 1 will they be able to submit a dossier in Denmark for this study. And then, ABC Pharma will have to wait between 52 and 83 additional days before Denmark delivers a clinical trial authorization.
If ABC Pharma is not ready to add Denmark when they click on the submit button, it means they will not be able to get clinical trial authorization in Denmark or any other additional EU country for 112 to 189 days.
The same problem will arise if the Sponsor wishes to file a substantial modification. This will also have to wait until the end of the assessment on Part I.
So, before clicking on the “submit” button, ABC Pharma would have to ensure good coordination to prevent blocking the beginning in an EU country for hundreds of days.
Three Strategies to Solve Potential Problems
To face this new challenge, ABC Pharma may think about different types of strategies:
Strategy 1: Submit Parts 1 and 2 in one EU country known for a fast review timeline. The timelines provided in the above scenario are the maximum length. Some EU countries are known to assess the clinical trial application faster than the maximum limit. Then, once Part 1 is approved by this country, ABC Pharma can add the other EU countries in waves. The addition of different EU countries will not be frozen anymore once the Part I is approved by at least one country.
Strategy 2: Delay the submission to be sure all the EU countries needed are included when clicking on the “submit” button.
Strategy 3: Cancel the application and restart the submission process in order to not block the addition of a new country for hundreds of days.
As mentioned in the title, coordination and careful upfront planning is needed to properly navigate the intricacies of the new EU Clinical Trials Portal.
To speak to a transparency expert, email info@mmsholdings.com.





