Project Renewal is an initiative established by the FDA to reassess outdated oncology drug products and modernize them.
The Oncology Center of Excellence (OCE) recently approved a labeling update for Genentech’s capecitabine tablet, known as Xeloda. In this labeling update, this decades-old drug (NDA 020896; approved in 1998) was approved for expansion from the prior indications in colon, colorectal, and breast cancer to include new indications, such as gastric, esophageal, or gastroesophageal junction cancer and pancreatic adenocarcinoma. Additional updates were made to several dosage regimens and information for safe administration and use, including patient counseling information.
This update is just one drug in a much larger initiative, known as Project Renewal, established by the FDA to reassess outdated oncology drug product labels and modernize them to the most up-to-date information and formatting.
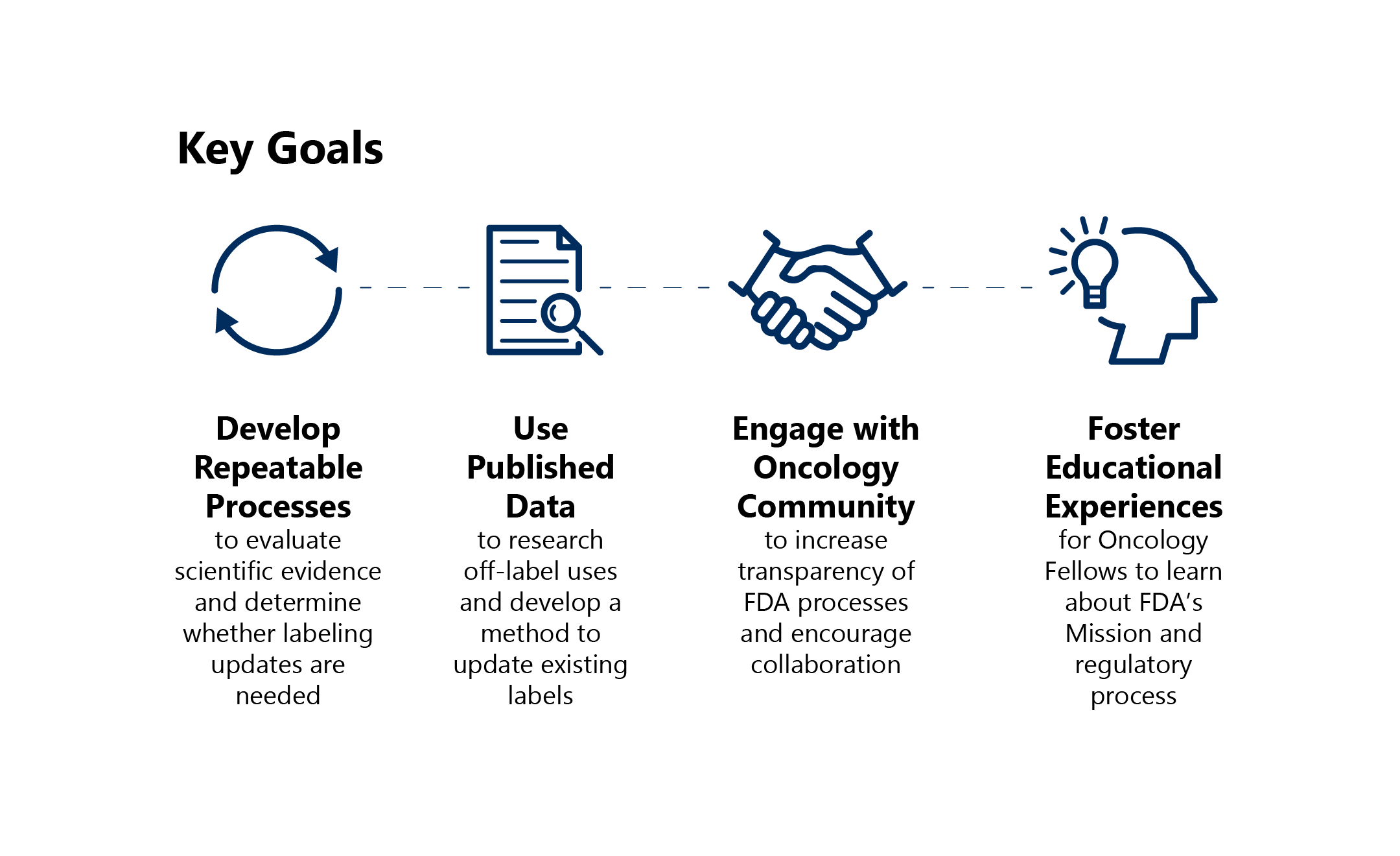
FDA’s goals for Project Renewal are:
Source1
The FDA is primarily exploring oncology drugs that are several decades old and are either not updated to the modern Physician Labeling Rule (PLR) or that have had significant use as an off-label drug in the US oncology community. 2
What labeling information is the FDA interested in and why?
Overall, the OCE is focused on all labeling information that could inform the effective and safe use of the drug. They are specifically interested in expanding to include new indications, updating the dosing regimen, and updating to follow the PLR format and text. This process is also intended to increase the general awareness of drug labeling as a source of information for those making healthcare decisions for patients with cancer.
As new (off-label) indications and dosing regimens emerge during real-world use, they are not always added to labels during the post-marketing phase. The older a drug is, the more common this practice seems to be. Labels are also sometimes not updated with other publicly available information regarding indications and dosing after approval.2
Additionally, PLR updates were meant to provide uniform formatting and to make it easier to locate pertinent information like drug interactions, dosage, etc. However, only BLAs, NDAs, and efficacy supplements approved on or after June 20, 2001, must adhere to the PLR in accordance with 21 CFR 201.56(d) and 201.57.3 While drugs approved prior to that date technically do not need to be updated to PLR, the “FDA strongly encourages all applicants to voluntarily convert the labeling of their drug products to the PLR format, regardless of the date of approval”.4
How will this be accomplished?
As a first step, the OCE has begun initial research into potential oncology products to identify standard off-label uses for those products, especially those included in Standard of Care recommendations or guidances. FDA also reaches out to the drug holders to initiate engagement in this effort.
Next, research team members and oncology fellows are onboarded to help evaluate evidence, and relevant, publicly available literature is summarized in a report. This evidence is discussed in a Labeling Evidence Evaluation Process culminates in a final product report, including information on labeling considerations and available evidence.
Finally, the FDA reviews the report, the labeling considerations are kept in a repository, and the findings are published. Lessons learned about the Project Renewal process are noted as well. 5
What’s next?
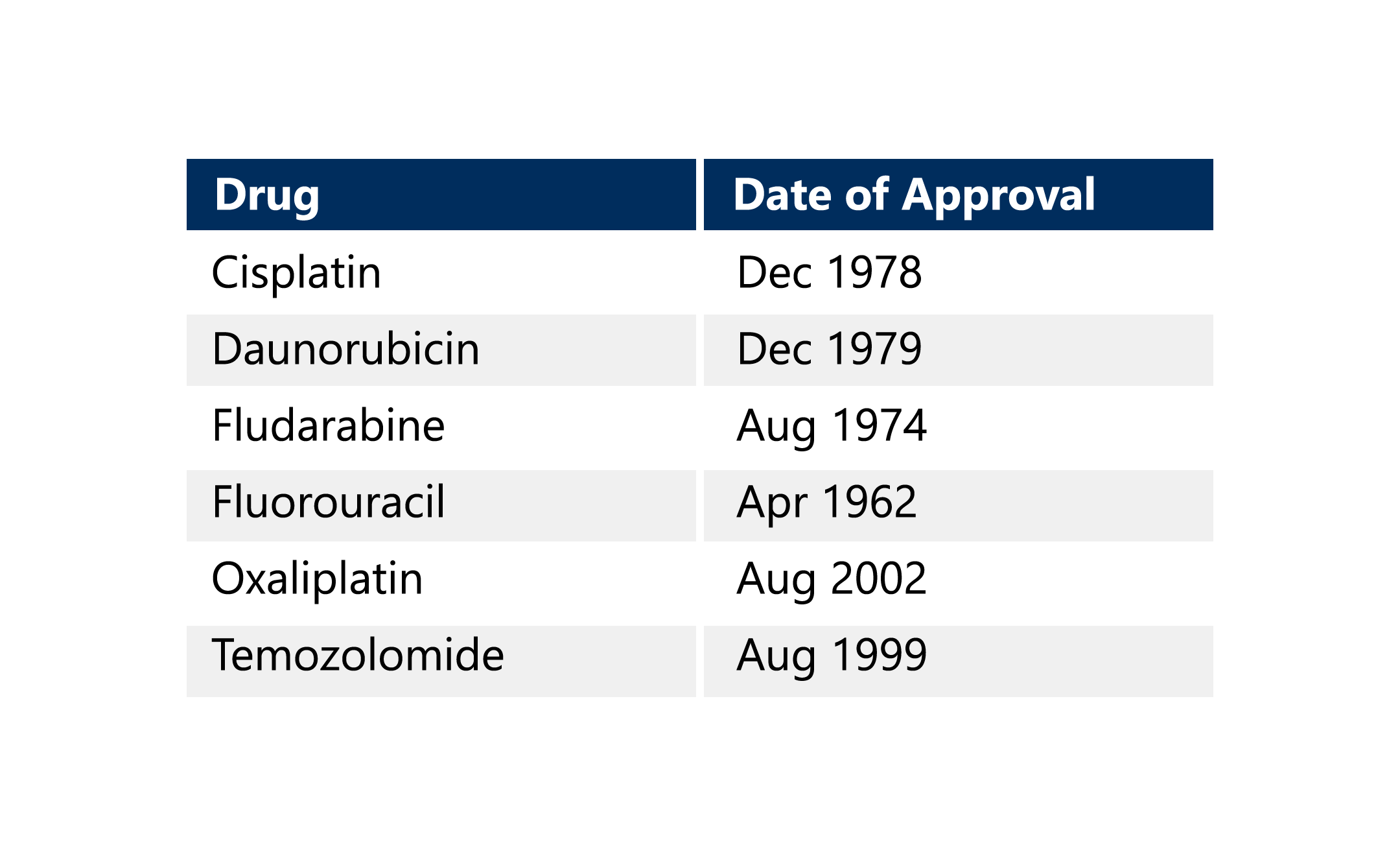
The Oncology Center of Excellence has already addressed capecitabine and will continue to work through its current Candidate Drug List. This list includes the following drugs. 5
If you have any questions about Project Renewal, please email info@mmsholdings.com to speak to an expert.
References
4 PowerPoint Presentation (fda.gov)