On 31 January 2022, the EU Clinical Trial Regulation 536/2014 (commonly known as EU-CTR) replaced the long-standing directive, the European Union (EU) Clinical Trial Directive 2001/20/EC (commonly known as EU‑CTD).
There are important changes in the EU-CTR, compared to EU‑CTD, that significantly impact the governance of clinical trials in the EU. This new regulation aims to improve efficiency, vigor, and transparency of clinical studies while ensuring the highest standards of safety for clinical trial participants in the EU.
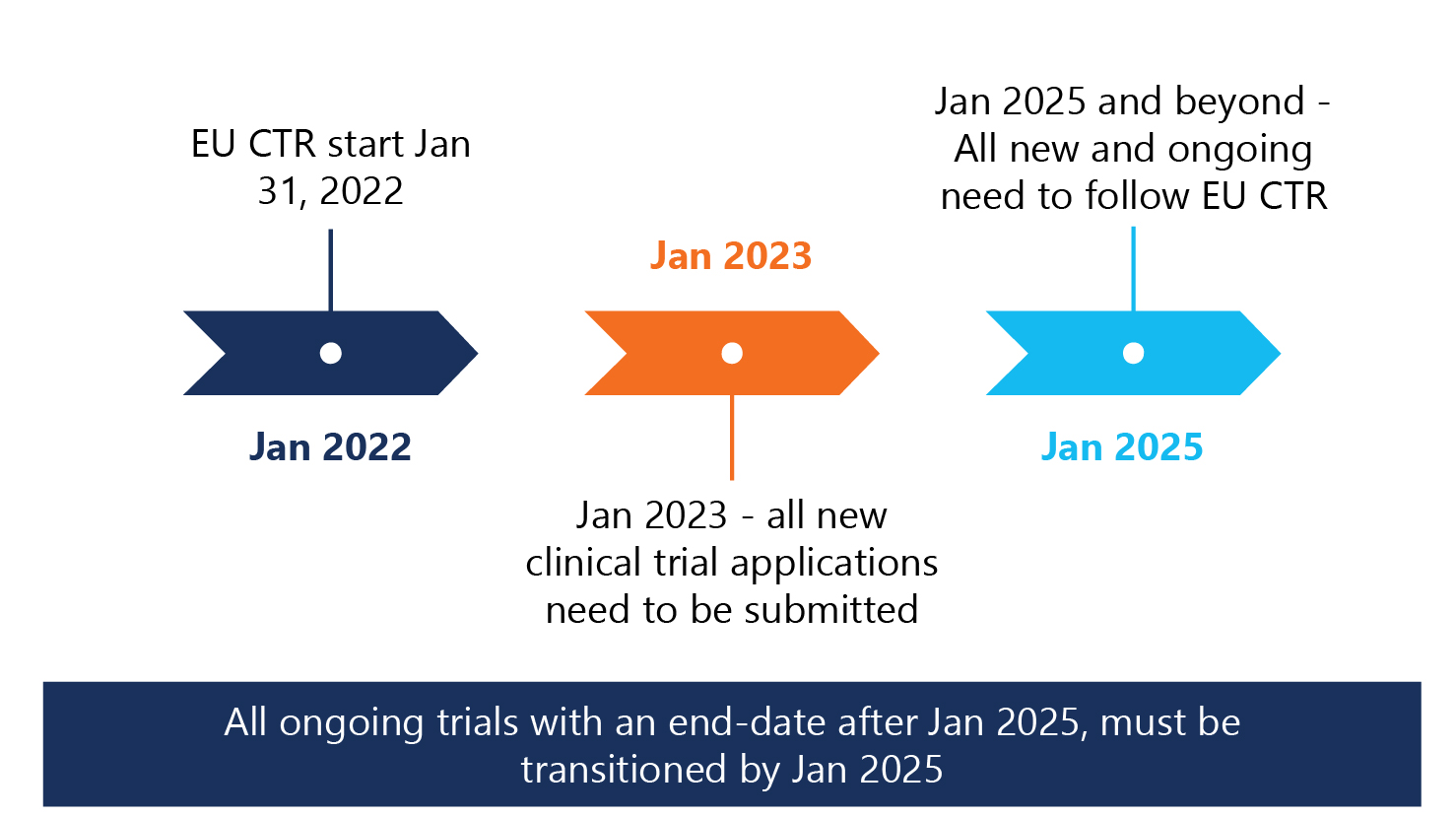
3-year phased implementation approach adopted
Per the EU-CTR, a 3-year phased implementation approach regarding clinical trial applications and the use of EU-CTD versus EU-CTR starting January 2022 was adopted.
The release of the Clinical Trial Information System (CTIS)
The release of a streamlined submission portal/database called the Clinical Trial Information System (CTIS) is a notable adoption under EU-CTR.
The CTIS is a portal allowing Sponsors and Health Authorities to manage all submissions and data under the EU-CTR by secure access. In addition, the CTIS supports transparency by providing open-access content for the general public. Patients, healthcare professionals, scientists, media, and other public members can now access most of the same clinical trial data.
Increased transparency with patients builds public trust in health research and clinical trials; previously, transparency has not been necessary until the product is approved.
Increased transparency for public awareness
With the release of EU-CTR, the new transparency rules aim is to increase public awareness of and information sharing among studies.
Publicly available documents include:
- Clinical Trial Application documents, including the application form and Parts I and II (including substantial modifications)
- Responses to requests for information
- Clinical trial dates including start/stop of trial, start/stop of recruitment
- Unplanned notifications such as urgent safety measures, trial pausing/stopping
- Results from the trial, including technical summary results, plain language (layperson) summary, and clinical study report (after marketing authorization decision)
Regardless of the decision made by the applicable Health Authority to approve or reject the CTA, the majority of clinical trial data and documents submitted via EU-CTIS will be publicly available, with the following exceptions (per Article 81 (4) of the Clinical Trials Regulation No. 536/2014):
- if the communication is confidential between EU member states regarding their assessment of the application
- to protect effective supervision of clinical trials
- when necessary to safeguard protected personal data and commercial confidential information
What is considered protected personal data and commercial confidential information?
| Protected personal data: | Information relating to an identified or identifiable person by one of more factors specific to their physical, physiological, mental, economic, cultural, or social identity. |
| Commercial confidential information: | Information not in the public domain or publicly available. Information, when disclosed, may undermine the economic interest of the Sponsor. |
The impact of the EU-CTR on clinical trials
Overall, the EU-CTR will increase the visibility of clinical trials – more trials will now be publicly disclosed than ever before. All clinical trials conducted in the EU will now be publicly disclosed. In addition, any data from a clinical trial that is to be included in an EU-CTR application (even if the trial is conducted outside of the EU) must be prospectively registered.
The EU-CTR increases the public access to information more than ever with the simple click of a button, and the public will be able to follow the progress of clinical trials in real-time.
The EU states have committed to this regulation to increase public knowledge and awareness of science and medicine.
What this means for Sponsors
Sponsors must be keenly aware of the new transparency landscape and what will be the impact of their trial’s documented information and results becoming publicly available; otherwise, the Sponsor might risk not only increased cost and burden but, more importantly, may impact the timeline of therapeutic development.
Sponsors that choose to adopt this regulation before EU-CTR takes full effect will lead the upcoming years in innovation and research.
By: Aaron Burr, Ph.D., Senior Medical Writer, Jennica Young, Ph.D., Clinical Trial Transparency Specialist, and Shaik Asma Umira, Pharm-D, Medical Writer
If you have questions about the EU-CTR, please email info@mmsholdings.com to speak to an expert.
View our webinar on the EU-CTR here.





