Plain language protocol synopsis (PLPS) is an important part in informing patients from the start of their clinical trial experience. On January 21, 2022, the European Medicines Agency (EMA) launched a new clinical trials website, Clinical Trials Information System (CTIS), and a new clinical trials regulation (EU) No 536/2014 came into effect. With this, a plain language protocol synopsis (PLPS) must be submitted as part of the initial application for a clinical trial.
According to the European Commission, one of the goals of this regulation (Regulation [EU] No 536/2014) is to ensure a greater level of harmonisation of the rules for conducting clinical trials throughout the EU. It introduces an authorisation procedure based on a single submission via a single EU portal, an assessment procedure leading to a single decision, rules on the protection of subjects and informed consent, and transparency requirements, including the plain language protocol synopsis (PLPS).
Protocols, in general, describe how a clinical trial will be carried out before any clinical trials begin, in detail. It has become difficult for patients to find clear and concise information about specific clinical trials when they search for research that is relevant to them. Study staff may not be equipped with Sponsor‑approved plain language tools to facilitate conversations with patients about a clinical trial.
This represents a missed opportunity to appropriately engage and inform patients from the start of their clinical trial experience. To build trust between the public and the researchers, one needs to be cognizant of how some studies/trials influence certain people or certain communities. This will help to elucidate complex theories among the public.
The plain language protocol synopsis (PLPS) is the answer to this.
A plain language protocol synopsis (PLPS) is important so that the general public can have an understanding of what researchers and others have planned for a particular clinical study. It will help anyone interested in the research to understand the project without all the details necessary in a full protocol and in lay language.
What is included in a plain language protocol synopsis (PLPS)?
Sponsors should include the information below in the plain language protocol synopsis (PLPS). A maximum of two pages to be submitted with the clinical trial application according to Annex I D24, including:
- EU trial number and full trial title
- Rationale
- Objective
- Main trial endpoints
- Secondary trial endpoints
- Trial Design
- Trial Population
- Interventions
- Ethical considerations relating to the clinical trial, including the expected benefit to the individual subject or group of patients represented by the trial subjects as well as the nature and extent of burden and risks.
Developing a plain language protocol synopsis (PLPS)
A plain language protocol synopsis (PLPS) should be created with the general public in mind. According to the Center for Plain Language, the average person reads at a7/8th grade reading level. And, much of the health information available today is communicated at a 10th grade level or higher.
If the clinical trial material is not understood by patients, this can limit patient engagement and recruitment during clinical research studies. This can also impact treatment adherence and lead to poorer health outcomes down the line.
To mitigate these risks, a plain language protocol synopsis (PLPS) should be developed that targets groups that may not have the same level of health literacy as medical professionals or scientists.
In the plain language protocol synopsis (PLPS), it is necessary to ensure that the information included is clear and true, written in simple language. Also, the language used should be unbiased, thorough, and complete. In addition to these we need to ensure no advertising or promotional information is used in the plain language protocol synopsis (PLPS). While drafting a plain language protocol synopsis (PLPS), consider some of the following best practices:
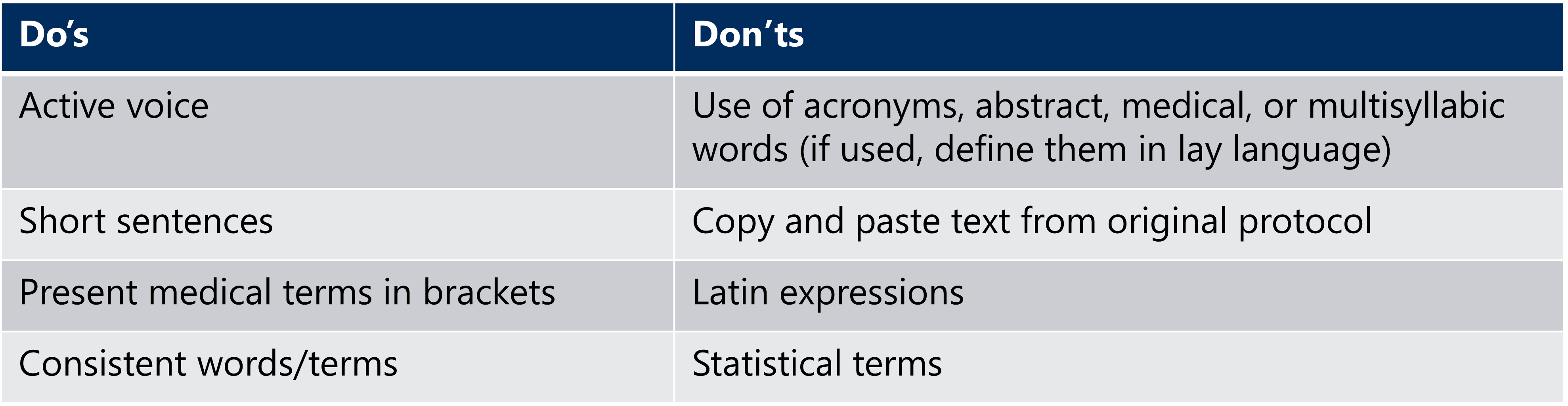
Making it available to the public
A plain language protocol synopsis (PLPS) should be made available on the internet and be read as a stand‑alone document.
The plain language protocol synopsis (PLPS) is required to be submitted with the Clinical Trial Agreement (CTA) as a part of a clinical study protocol or a separate document (i.e., when it is submitted in different language versions) in CTIS according to Regulation (EU) No 536/2014 Annex I D.24.
This document will be authored in English and then translated into the local language of each country where the study would take place.
MHRA Requirements – ISRCTN Registry
On top of publishing the plain language protocol synopsis (PLPS) in CTIS, the Medicines and Healthcare products Regulatory Agency (MHRA) in the UK requires International Standard Randomised Controlled Trial Number (ISRCTN) registry.
For this registry, the plain English summary describes the research to the non-expert public and should be written in easily understood plain English in under or around 1,000 words.
The following information should be included for this plain language summary in the UK per MHRA requirements:
- Background and Study Aims
- Who can participate?
- What does the study involve?
- What are potential risks/benefits?
- Where is the study run from?
- When is the study starting and what is the expected duration?
- Who is funding the study?
- Who is the main contact for the study?
By building and refining the plain language protocol synopsis (PLPS) development process, Sponsors can ensure that they are communicating the essential information in a way that is easily understood by the target audience, while complying with relevant regulatory requirements. Developing a plain language protocol synopsis (PLPS) for each clinical trial is essential for the inclusion of all people and participants that do not have the same level of understanding as medical professionals and scientists.
For additional information or questions on developing a plain language protocol synopsis (PLPS), Please click here to connect with the right resource to respond.
Authored by:
Nitha Chandran, Principal Medical Writer, Regulatory and Medical Writing.
Amee Atha, Senior Medical Writer, Regulatory and Medical Writing.





